Abstract
Background: Standard induction therapy for Multiple Myeloma (MM) is triple combination therapy based on three classes of drugs: proteasome inhibitors, immunomodulators and steroids. Despite its notable efficacy, bortezomib has serious issues of peripheral neuropathy (PN) with incidence of grade ≥ 3 PN reported between 2% to 23%. A novel proteasome inhibitor, carfilzomib (CFZ) has high selectivity and minimal off-target adverse effects including lower rates of PN. CFZ has been approved for treatment of relapsed and refractory multiple myeloma (RRMM) as single agent as well as in combination with lenalidomide and/or dexamethasone. We conducted comprehensive literature search to summarize efficacy, dosing and toxicity profile of CFZ in newly diagnosed and relapsed MM.
Methods: Database search using PubMed, EMBASE, Cochrane library, Scopus, Web of Science, CINAHL, and Clinicaltrials.gov was performed on 6/5/2017. Phase II/III clinical trials from last 10 years and focusing on CFZ as primary drug therapy were included.
Results: Literature search identified a total of 1839 articles. Twenty-six articles met the inclusion criteria (15 in newly diagnosed multiple myeloma (NDMM) and 11 in RRMM group) with a total of 5980 patients (4205 in RRMM and 1775 in NDMM group).
For heavily pre-treated RRMM patients with median 5 prior lines of therapy, 20/27 mg/m2 of single agent CFZ produced outcomes ranging from 3.5 to 5.1 months of progression free survival (PFS), 16.7% to 23.7% of overall response rate (ORR) and 16% to 18.3% of partial response (PR). Higher dose 20/56 mg/m2 of CFZ in Kd (carfilzomib + dexamethasone) regimen produced PFS of 4.1 months and ORR of 55% in patients with 5 prior lines of therapy. Even higher 20/70 mg/m2 dose of CFZ in Kd regimen produced ORR of 77% and PFS of 12.6 months in patients with 1 prior therapy. ENDEAVOR and ASPIRE trials reported PFS of 18.7 months with Kd vs 9.4 months with Vd (bortezomib and dexamethasone) and 26.3 months with KRd (carfilzomib, lenalidomide and dexamethasone) vs 17.6 months with Rd (lenalidomide and dexamethasone) respectively, in patients with median 2 prior lines of therapy.
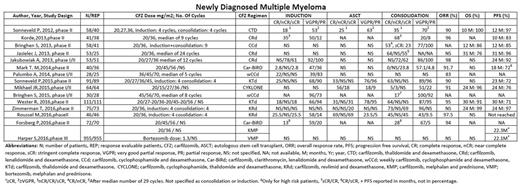
CFZ in front line setting achieved high response rates. In NDMM, most commonly studied regimen was CFZ in combination with lenalidomide and dexamethasone (KRd) with 4 phase II and 1 phase I/II trial (n= 268). Their outcomes range from 96% to 98% ORR, 83% to 97% overall survival (OS) and 92% to 97% of 2-year PFS. Likewise, outcomes of other CFZ based regimens, 6 phase II and 3 phase I/II trials (n= 552) range from 72% to 97% ORR. Data on overall survival (OS) and progression free survival (PFS) was either not reported or reported at 2 or 3-year interim analysis. Observed PFS in a recent phase III trial comparing CFZ, melphalan and prednisone (KMP) with bortezomib, melphalan and prednisone (VMP) was 22.3 months vs 22.1 months respectively.
Most common grade ≥3 AE were hematologic. Incidences of non-hematologic grade ≥ 3 AE include heart failure (3.4% to 20%), acute renal failure (3.4% to 9%), HTN (4.3% to 25%) and PNP (<1% to 1.1%). ENDEAVOR reported significantly higher incidence of ≥2 PNP in bortezomib group (32%) vs 6% in carfilzomib group. ENDEAVOR trial using serial echocardiograms reported no increased risk of cardiotoxicity and ventricular dysfunction with CFZ vs bortezomib.
Conclusion: CFZ demonstrates comparable if not higher efficacy to bortezomib with much favourable adverse effect (AE) profile. Most common grade ≥ 3 AE were hematologic that usually don't require dose changes or discontinuation of therapy. High incidence of grade ≥3 HTN deserves attention and underscores the importance of serial BP monitoring. In RRMM, CFZ has documented efficacy with standard 20/27mg/m2 dose. Further large-scale trials are needed to study benefit-to-risk profile of 20/56 and 20/70mg/m2 dose of CFZ vs standard 20/27mg/m2 dose.
All trials except one on CFZ for NDMM were phase II clinical trials with data available mostly as abstracts. So, there was insufficient data available to compare studies. Deep, rapid and sustainable response with KRd with lower incidence of crippling PN strongly supports extension of KRd therapy to frontline setting. More data from phase III trials is needed for head to head comparison of KRd vs other regimens like bortezomib, lenalidomide and dexamethasone (RVd) for treatment of NDMM. CFZ should be tested in combination with other available drugs like daratumumab and pomalidomide.
Abraham: Janssen Oncology (Johnson & Johnson, LLC): Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal